Cover Gallery
We acknowledge the importance of presenting our work, which is why we invite you to peruse our gallery showcasing the covers of our publications. We hope you find them inspiring!
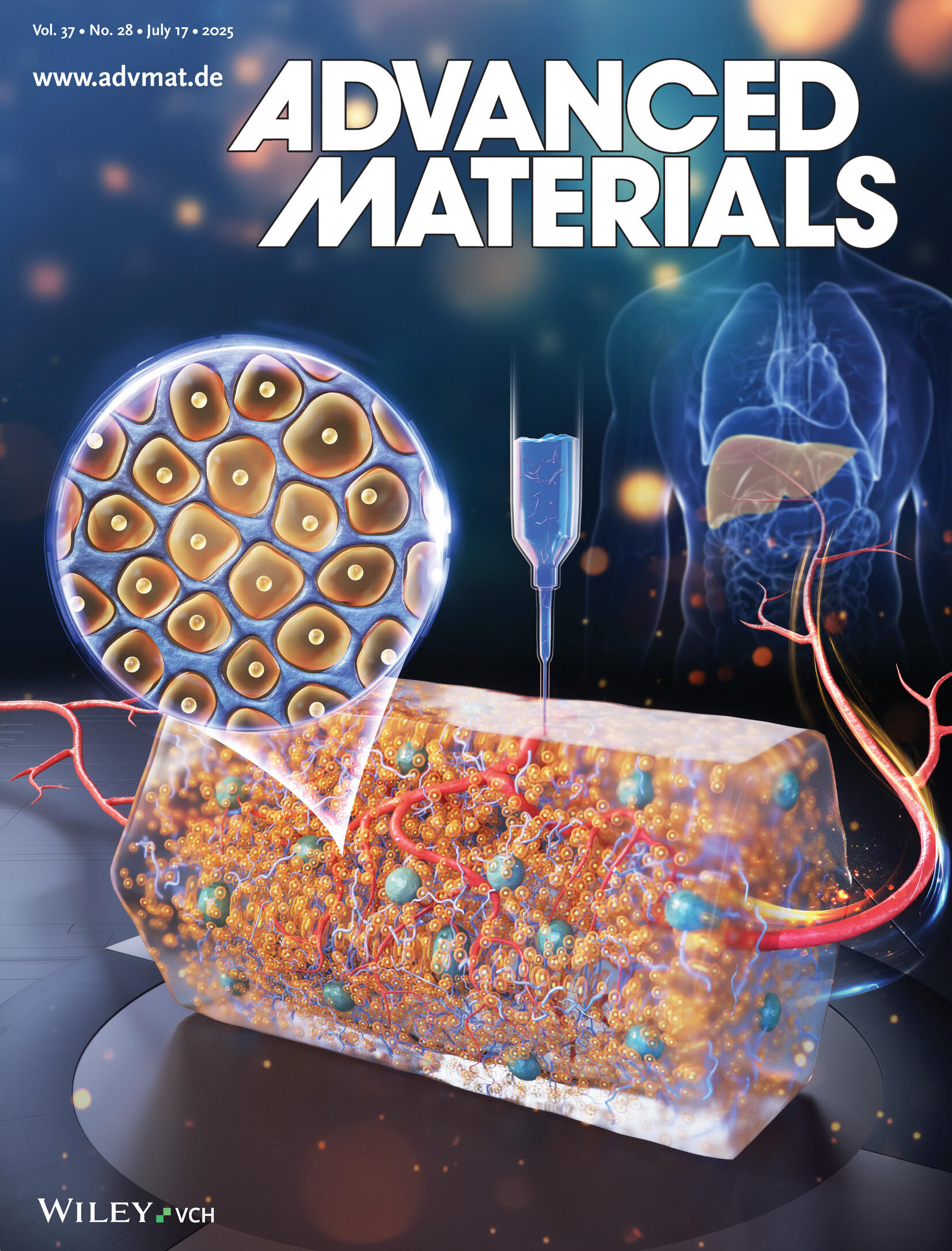
In article number 2413940, Qi Gu and co-workers introduce a heterogeneous microgel–hydrogel hybrid designed to carry high-density hepatocytes while supporting the embedded bioprinting of hierarchical vascular structures. The thick, vascularized constructs, bioprinted with hepatocytes, enable swift functional recovery and prolonged survival of rats following 85% hepatectomy. This study presents an innovative approach to enhance regenerative therapy outcomes through advanced bioprinting and vascular integration techniques.
In this article, Minxuan Jia, Tingting Fan, Tan Jia, Xin Liu, Heng Liu, and Qi Gu harness advanced 3D bioprinting to orchestrate the temporal and spatial regulation of biomimetic vascularization in skeletal muscle tissues. By co-constructing muscle and endothelial cells, they recapitulate the natural interplay between myotubes and their nourishing vasculature, which not only enhances myotube assembly but also accelerates the formation of early vascular networks essential for oxygen and nutrient transport. This innovative approach yields physiologically aligned, large-scale muscle constructs that closely mimic native tissue architecture while unveiling novel insights into the dynamics of multicellular interactions during tissue maturation. Consequently, their work lays a robust foundation for future regenerative therapies by charting promising pathways for engineering complex, functional tissues and organs.
Beyond these core findings, the study invites further exploration into refining bioprinting parameters and cell co-culture strategies to optimize the vascular integration process, potentially broadening the scope of applications in tissue engineering and regenerative medicine.
Gu et al. introduce the “3P Model”, a machine learning framework for evaluating bioartificial organ function, focusing on liver tissue engineering. This innovative approach analyzes extensive experimental data to identify key parameters affecting organ functionality, such as albumin and urea secretion. The model’s predictive capabilities aim to optimize culture conditions and improve the functional sustainability of bioartificial livers. This research marks a significant advancement in standardized organ chip manufacturing, promising enhanced efficiency and scalability in the fabrication of bioartifi cial organs.
In article 2300685, Chen, Gu, and co-workers explore the progress in human microphysiological constructs and systems. They harness technologies such as stem cells, biomaterials, bioprinting, biosensors, organ chips, and artificial intelligence. These advancements offer substantial promise for gaining insights into the human body and applying them to the study of disease mechanisms, drug development, and organ repair.
In this article, Zili Gao, Jia Guo, Bo Gou, and co-workers introduce novel droplet microfluidics-derived, laminin-modified microcarriers with tunable stiffness to mimic the biomechanical cues of blastocyst trophoblast cells, thereby promoting the through-interface movement of mouse trophoblast stem cells. The biomimetic microcarriers display excellent cytocompatibility and significantly enhance cell adhesion, expansion, and invasion, yielding a robust in vitro model of embryo implantation. This study paves the way for deciphering the mechanotransductive mechanisms that regulate cell migration and implantation dynamics.
In article number 2212114, Wenguo Cui, Qi Gu, Ming-Zhu Zhang, and co-workers introduce novel cartilage lacuna-inspired microcarriers with geometric constraints to repress chondrocyte dedifferentiation by inhibiting Wnt signaling. The biomimetic microcarriers display favorable cytocompatibility and drive robust hyaline chondrocyte-derived neocartilage formation. This study paves the way for deciphering the geometrical insights into the interactional mechanism of mechanotransduction in regulating cell fate.
In vitro culture of embryos is a useful tool to explore the mysteries of life. As the natural platform for embryo development, the uterus inspires the building of in vitro culture conditions. In article number 2202282 by Qi Gu, Hongmei Wang, Shutao Wang, and co-workers, particularly designed collagen is grafted onto polydimethylsiloxane to simulate the moduli of the endometrium and uterine horn and the microstructure of the endometrium during mouse pregnancy. The uterus-inspired niche microenvironment controls the migration and assembly of extraembryonic tissues, simulates embryo implantation, and ultimately promotes embryo development to early organogenesis.
In this article, Jia Guo, Yuanyuan Li, Zili Gao, and co‐workers harness 3D printing technology to fabricate anisotropic microporous PDMS scaffolds that recapitulate key aspects of the uterine microenvironment and promote in vitro embryonic development. By judiciously tuning the advancing angle between printed layers, they engineered scaffolds with well‐controlled porosity and wettability that modulate embryo–substrate interactions. Notably, the 30° and 60° configurations yield moderate cell–scaffold attachments, fostering optimal epiblast (OCT4-positive) formation while restraining excessive extraembryonic tissue growth. This robust system not only supports sequential developmental milestones—including the migration of T-positive and FOXA2-positive cells reminiscent of gastrulation—but also paves the way for deciphering the mechanotransductive cues regulating embryo implantation and potential applications in uterine regeneration.
In article number 2101405 by Shu Wang, Xiongfei Zheng, Qi Gu, and co-workers, a bioprinting methodology with multistage-temperature-control is developed, producing centimeter-scale vascularized soft tissues containing branched endothelialized vasculatures and well-formed 3D capillary networks. It ensures cellular activities and then mimics mature and functional liver tissue.
The cover artwork for article number 1700175 represents novel tissue engineering by 3D bioprinting human induced pluripotent stem cells to create cell-laden constructs with the capacity to form ectodermal, endodermal and mesodermal tissues (exemplified by neuronal, lung and muscle cells) and therefore all tissues of the body. This method is introduced by Gordon G. Wallace, Jeremy M. Crook, and co-workers.
On page 1429 G. G. Wallace, J. M. Crook, and co-workers report the first example of fabricating neural tissue by 3D bioprinting human neural stem cells. A novel polysaccharide based bioink preserves stem cell viability and function within the printed construct, enabling self-renewal and differentiation to neurons and supporting neuroglia. Neurons are predominantly GABAergic, establish networks, are spontaneously active, and show a bicuculline induced increased calcium response.
In this article, Qi Gu, Jie Hao, YangJie Lu, Liu Wang, and Qi Zhou present a comprehensive review of three-dimensional bio‐printing, showcasing how advances in 3D printing technology are poised to reshape medical applications. They outline the revolutionary shift driven by the unique capabilities of 3D printing—such as unmatched customization, flexibility, and high resolution—which have already transformed sectors like automotive and aerospace, and are now being leveraged for biomedical innovation. The authors discuss current applications ranging from hard tissue reconstruction to the development of sophisticated drug-delivery systems, highlighting the potential of fabricating complex three-dimensional structures that incorporate living cells and bioactive agents. While the technology has made significant strides, the review also identifies the inherent challenges in engineering fully functional tissues and organs, marking a crucial frontier for future research in regenerative medicine and tissue engineering.